Cook IVC Filter Bellwether Trials Start in 2016
 Bellwether selection has begun in the coordinated proceedings involving Cook vena cava filter lawsuits. Ten cases have been selected by representatives for the plaintiffs and the defendants, which will now go through case-specific discovery.
Bellwether selection has begun in the coordinated proceedings involving Cook vena cava filter lawsuits. Ten cases have been selected by representatives for the plaintiffs and the defendants, which will now go through case-specific discovery.
Four of those cases will then be identified as bellwether trials, which are scheduled to begin later next year.
About IVC filters
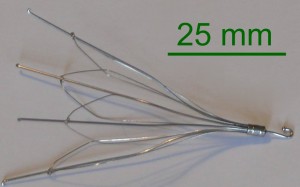
All of the IVC filter lawsuits, which were consolidated into coordinated proceedings last November, involve allegations that retrievable vena cava filters manufactured by Cook Medical led to serious complications. The small, spiderlike filters were created for implantation into the inferior vena cava (IVC) to stop blood clots from traveling from veins in the lower body to the lungs, where they can lead to a potentially deadly event known as a pulmonary embolism. The filters were recommended for patients at risk for pulmonary embolism that were unable to take blood thinning medications or found the medications to be ineffective.
Unfortunately, reports of serious side effects involving the filters have also been reported. These complications include migration of the devices, perforation of the IVC, and device fracture, which sends small particles from the filter traveling through the bloodstream. The problems can lead to long-term organ damage and the need for surgery to remove filter particles and repair damage.
In May 2014, the FDA issued a safety communication regarding IVC filters. The agency noted the potential complications of these devices, including migration, filter fracture and perforation of the IVC. The FDA urged medical providers to monitor patients with IVC filters closely and remove the filters as soon as the threat of pulmonary embolism passed, after determining that the risk of complications may increase the longer the filter remained in the IVC.
Cook IVC filter MDL status
As the number of lawsuits against Cook Medical continued to grow, multidistrict litigation for current cases was established in November 2014. Multidistrict litigation, or an MDL, coordinates cases with similar statements of fact for the purpose of streamlining early trial proceedings. This action serves the convenience of all parties involved, by eliminating duplicate discovery and preventing conflicting rulings in different courts. The MDL was assigned to U.S. District Court for the Southern District of Indiana, where they are now overseen by U.S. District Judge Richard L. Young.
According to the website for the court, there are now more than 130 cases pending in the MDL. In July, Judge Young signed a case management order outlining how cases were to be selected for bellwether trial discovery. Each side identified five lawsuits, which were submitted to the court on October 5. Over the next six months, depositions and written discovery will be conducted on these 10 lawsuits, which will be followed by the selection of four cases as bellwether trials. The first bellwether trial is slated to begin sometime after September 15, 2016.
However, Judge Young’s case order also included directions for plaintiffs to submit a statement of special damages and make a settlement demand by November 2, 2015. Cook Medical will then have 60 days to respond to the statement and settlement proposal, which could avoid the need for the majority of these cases to go to trial. If the cases are not resolved at this time, bellwether proceedings will continue as planned.
Coordinated litigation for Bard claims
Cook Medical is not the only company facing litigation involving their retrievable IVC filters. Bard has also been the subject of numerous lawsuits involving their own vena cava filters. In August 2015, the U.S. Judicial Panel on Multidistrict Litigation recommended the coordination of Bard IVC filter lawsuits into an MDL as well. That coordination has been assigned to U.S. District Court in Arizona, where it will be overseen by U.S. District Judge David G. Campbell.
- U.S. District Court, Southern District of Indiana, MDL No. 2570 in re: Cook Medical, Inc., IVC Filters Marketing, Sales Practices and Products Liability Litigation, http://www.insd.uscourts.gov/mdl-case-information
- U.S. District Court, Southern District of Indiana, Case Management Order #9, http://www.insd.uscourts.gov/sites/insd/files/MDL%202570%20Case%20Mgmt%20Order%209.pdf
- FDA, Removing Retrievable Inferior Vena Cava Filters: FDA Safety Communication, http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm396377.htm
- Medscape, Safety and Effectiveness of Inferior Vena Cava Filters Used to Protect against Pulmonary Embolus: A Technology Assessment, http://www.medscape.com/viewarticle/744331_2


 Resources
Resources
 Resources
Resources
