Zimmer Hip Lawsuit Filed in New Jersey District Court
In yet another lawsuit filed in the Multi-District Litigation (MDL) against Zimmer – which is steadily expanding – an injured plaintiff is seeking redress for his injuries following the malfunction and eventual revision surgery of his Zimmer Durom Cup hip implant system.
MDL No. 2158 was commenced in 2013 and is currently consolidated in New Jersey before Judge Susan D. Wigenton. As of January 15, 2015, it contains 341 pending cases, each alleging major problems and injuries associated with the Durom Cup hip implant system. The most recent plaintiff’s allegations are near-identical to those raised by the hundreds of other orthopedic patients affected by the Durom Cup model, and the plaintiff is seeking a fair and adequate compensation amount to help equalize the loss incurred as a result of a defective and dangerous medical product.
Details of Preskins v. Zimmer Holdings, Inc.
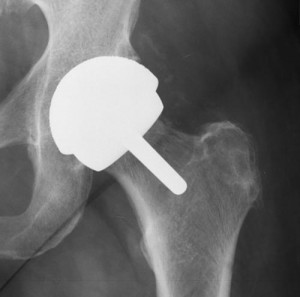
The plaintiff’s experience with the Durom Cup began upon the surgical implantation of the device in his left lip in March, 2007. Since then, the plaintiff alleges to have endured significant and enduring pain, affecting his ability to do household chores or to work.
Part of the plaintiff’s issues with the Durom Cup model, which are not unlike similar experiences had by other Zimmer hip replacement patients, involve the introduction of toxic metals and fragments into the blood stream. The plaintiff points out that the Zimmer Cup products are sprayed with a “highly engineered” coating designed to help reduce the occurrence of rejection by the body upon introduction of the hip system post-surgery.
This implant is designed to allow new bone to grow over and within the implant. However, the plaintiff experienced the opposite phenomenon, and endured episodes wherein the implant would pop loose in the pelvic socket, which can cause damage to surrounding bone and tissue.
The plaintiff contends that Zimmer, the maker of the Durom Cup, knew for years that it could cause painful injuries in patients, but continued to market and sell the products nonetheless. At one point, the Durom Cup model was the highest-selling hip replacement system in the world, with global sales topping $6 billion. It wasn’t until 2008, however, that the company finally admitted to possible problems with the system, and began initiating warnings to practitioners and patients.
Plaintiff alleges multiple causes of action against Zimmer
As with any product liability action, the MDL against Zimmer includes allegations that the company knew or should have known that its product was either defectively designed or improperly manufactured – and it failed to adequately warn consumers of the known risk.
The plaintiff points to several reports by top physicians, as well as warnings issued by the FDA, confirming that Zimmer had knowledge of the issues with the Durom Cup and failed to act. More specifically, top orthopedic surgeons revealed that over a one-year period, up to 23 percent of Durom Cup implantations failed.
The plaintiff alleged the following causes of action against Zimmer:
- Failure to warn or instruct patients as to the dangers with the Durom Cup
- Defective design
- Failure to adhere to proper manufacturing quality controls
- Negligence
- Breach of implied and express warranties
- Negligent and intentional misrepresentation
- Fraud
- Unfair and deceptive trade practices
- Unjust enrichment
The Zimmer hip lawsuit seeks enhanced compensatory damages to compensate for Zimmer’s reckless disregard of human life and suffering. It also seeks compensation for non-economic loss, including pain and suffering, mental anguish and emotional distress, as well as attorneys’ fees, costs and expenses included in the litigation.
- FDA.gov, Metal-on-Metal hip implants, http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/MetalonMetalHipImplants/ucm241770.htm
- Jpml.uscourts.gov, Transfer Order, http://www.jpml.uscourts.gov/sites/jpml/files/MDL-2158-Tag-Along_Transfer-01-13.pdf
- Jpml.uscourts.gov, January 2015 MDL docket, http://www.jpml.uscourts.gov/sites/jpml/files/Pending_MDL_Dockets_By_District-January-15-2015.pdf


 Resources
Resources
 Resources
Resources