Study Considers Factors in Mirena IUD Risks
As thousands of lawsuits mount across the country over Mirena IUD risks, a new study by the American College of Obstetrics & Gynecology focuses on a woman’s age and number of pregnancies as possible factors in IUD expulsion. The three-year study compared these risk factors in women implanted with hormonal and copper IUDs.
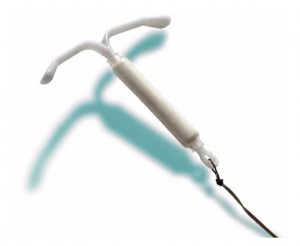
The Mirena IUD is a t-shaped piece of plastic that, once inserted, slowly releases progestin in order to interrupt fertility, thereby preventing pregnancy. Despite being the only hormonal IUD approved by the U.S. Food and Drug Administration (FDA), Mirena has been named in roughly 47,000 reports of IUD problems. About half of the reports relate to IUD expulsion. Some of the associated problems include pain, increased bleeding, little bleeding or amenorrhea, pregnancy, and even uterine perforation from Mirena migration.
Age a key factor in Mirena IUD risks
The new study examined the cases of over 5,000 women who had received IUDs. Over the three years of the study, a little over 10% of the devices were expelled. The rate of expulsion was the same regardless of whether the device was hormonal or copper.
Two other factors the researchers considered were maternal age and parity – the number of pregnancies she had already had. There was an association between expelled IUDs and age; expulsion was more common in IUD users aged 14-19 than in older women. At first glance, it appeared that women who had never experienced a pregnancy had a lower expulsion rate. However, after adjusting for other factors, there was ultimately no difference in expulsion rates between women who had and had not been pregnant before.
Mirena faces FDA, lawsuits
As reports of Mirena IUD side effects grew, the FDA stepped in, issuing a warning letter to Mirena’s manufacturer, Bayer. The December 2009 letter accused Bayer of failing to fully disclose the Mirena IUD risks while at the same time overstating the device’s benefits.
According to the FDA, some of the problems that Bayer did not fully disclose include:
- Increased risk of infections
- Risk of pregnancy while using the device
- Risk of miscarriage or even loss of fertility if a woman becomes pregnant while implanted with the IUD
- The need to check regularly to confirm that the device was still in place and had not been expelled or migrated to another part of the body
These risks at the center of the FDA warning are the same types of risks that led thousands of women to file product liability lawsuits against Bayer. The lawsuits have been filed in both federal and state courts and many have been consolidated in federal multidistrict litigation (MDL) or a similar type of state case consolidation.
The federal Mirena MDL is proceeding before Judge Cathy Seibel of the U.S. District Court for the Southern District of New York. The MDL consists of over 600 Mirena IUD lawsuits. The claims are following a uniform discovery and pretrial schedule to promote efficiency and consistency among the many cases. The initial round of cases, known as bellwether cases, are expected to head to trial starting in March 2016.
- U.S. National Library of Medicine National Institute of Health, Association of Age and Parity With Intrauterine Device Explulsion, http://www.ncbi.nlm.nih.gov/pubmed/25198262
- United States Judicial Panel on Multidistrict Litigation, In Re: Mirena IUD Products Liability Litigation, Transfer Order, http://www.jpml.uscourts.gov/sites/jpml/files/MDL-2434-Tag-Along-Transfer-05-14.pdf


 Resources
Resources
 Resources
Resources