After Mirena Removal, Plaintiff Claims Permanent Injuries
 A woman who endured surgical Mirena removal has filed a lawsuit against Bayer Healthcare Pharmaceuticals, Inc., demanding compensatory damages for her medical expenses, physical injuries, and pain and suffering. Sonja Zimmerman filed her lawsuit against Bayer on September 3, 2015 in the U.S. District Court for the District of Minnesota.
A woman who endured surgical Mirena removal has filed a lawsuit against Bayer Healthcare Pharmaceuticals, Inc., demanding compensatory damages for her medical expenses, physical injuries, and pain and suffering. Sonja Zimmerman filed her lawsuit against Bayer on September 3, 2015 in the U.S. District Court for the District of Minnesota.
Although Zimmerman is a resident of Pennsylvania, the lawsuit states that the Minnesota venue is appropriate in this case because the defendants conduct substantial business there, because there is a complete diversity of citizenship, and because the plaintiff is demanding damages in excess of $75,000. The plaintiff is demanding a trial by jury.
About the Mirena IUD
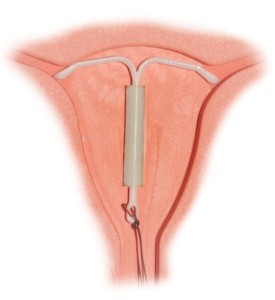
Mirena is an intrauterine device (IUD). It is a small, flexible piece of plastic that a healthcare provider can insert into a woman’s uterus for the prevention of pregnancy. It works by releasing levonorgestrel, a type of progestin, to thicken the cervical mucus. This prevents sperm from reaching the egg. Mirena also partially suppresses the ovulation process and makes the uterine lining thinner to prevent an egg from implanting. The device can remain in the uterus for up to five years.
While Mirena has become a popular method of long-term birth control in the U.S., a growing number of plaintiffs have filed lawsuits against Bayer Healthcare, alleging that the device led to serious complications and that the defendants failed to warn of these risks. Specifically, the defendants are accused of stating that migration of the device may occur at the time of insertion, but failing to state that the device may spontaneously migrate at any time after insertion.
Spontaneous migration and Mirena removal
Zimmerman’s Mirena lawsuit states that the 26-year-old woman had the IUD inserted by a healthcare provider in January of 2011. The procedure was apparently well tolerated and Zimmerman did not report any symptoms that might be consistent with uterine perforation at the time of the insertion. In the fall of 2013, the plaintiff underwent an ultrasound exam, which revealed that the IUD was not in its proper place in the uterus. Zimmerman later underwent an x-ray to locate the device. It was found in the right pelvis. Just a few days later, she had to undergo laparoscopic surgery to remove the device.
Normally, in the event that Mirena does not migrate and perforate the uterus, a healthcare provider could remove the device from the uterus without surgery.
Zimmerman demands that the defendants be held liable for the injuries that she claims were serious and life-threatening. Her lawsuit lists abdominal pain, surgical removal, hospitalization, medical therapy, medical monitoring, and continuing treatment among her damages.
Bayer accused of aggressive marketing tactics
Like many other plaintiffs who have filed Mirena lawsuits against Bayer Healthcare, Zimmerman contends that the company engaged in aggressive marketing tactics. The complaint notes that the defendants were contacted in 2009 by the Department of Health and Human Services’ Division of Drug Marketing, Advertising, and Communications (DDMAC). This communication was in regards to the defendants’ consumer-directed Mirena Simple Style Statements Program, which involved representatives visiting private homes to give live presentations to groups of women.
During these presentations, the representatives allegedly claimed that Mirena would increase intimacy, romance, and emotional satisfaction, along with making its users “look and feel great.” DDMAC noted that these claims were unsubstantiated and that the defendants did not properly warn consumers of the risks of Mirena.
- Mayo Clinic, Mirena (hormonal IUD), http://www.mayoclinic.org/tests-procedures/mirena/basics/definition/prc-20012867
- FDA, Memo to Bayer Healthcare, http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/EnforcementActivitiesbyFDA/WarningLettersandNoticeofViolationLetterstoPharmaceuticalCompanies/UCM197229.pdf


 Resources
Resources
 Resources
Resources