Mirena Lawsuit Filed Alleging Organ Perforation
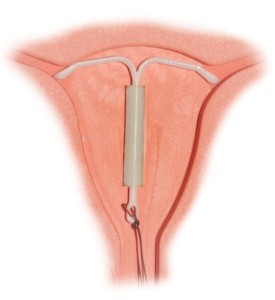 A plaintiff has recently filed a Mirena IUD lawsuit in Massachusetts court over serious complications allegedly connected to the implantation of the popular birth control device. Lawyers for Carolyn Ferrara filed the case (# 3:15-cv-30045) on March 19 against Bayer Healthcare in the U.S. District Court, District of Massachusetts, Western Division.
A plaintiff has recently filed a Mirena IUD lawsuit in Massachusetts court over serious complications allegedly connected to the implantation of the popular birth control device. Lawyers for Carolyn Ferrara filed the case (# 3:15-cv-30045) on March 19 against Bayer Healthcare in the U.S. District Court, District of Massachusetts, Western Division.
The complaint details the circumstances that purportedly led to complications: In an uncomfortable, but otherwise uneventful session, the device was inserted into the plaintiff on Aug. 18, 2011 at Northern Berkshire Healthcare by Susan J. Yates. Subsequently, Ms. Ferrara had to undergo laparoscopy to remove the device on Dec. 13, 2013 at North Adams Regional Hospital because it had perforated the “anterior rightward aspect of pelvis.”
According to the complaint, the use of the device and the subsequent surgery meant that the plaintiff “developed severe pain, suffered from infection, and underwent numerous procedures.”
Complaint alleges severe complications
The claim asserts that the defendant provided inadequate testing of the product, which they represented as safer and more effective than it was. According to the complaint, “Defendants have a history of overstating the efficacy of Mirena while understating the potential safety concerns.”
While relatively minor side effects (not mentioned in advertising, according to the complaint) include “weight gain, acne, and breast pain or tenderness,” other more serious side effects were also possible. The complaint goes on to describe how one Mirena advertising campaign “omitted information about serious conditions, including susceptibility to infections and the possibility of miscarriage if a woman becomes pregnant” while using the device.
Like the Massachusetts plaintiff, many women have brought suit against Bayer over issues related to migration of the device and perforation of the uterus and other organs or abdominal areas. The complaint in question, however, notes that the Mirena label “describes migration as uncommon event.”
Mirena IUD lawsuit one of thousands
The Mirena IUD (or IUS) was cleared by the FDA in December of 2000 and estimates cited in the complaint suggest that over 2 million women in the U.S. now use the device. The recently filed lawsuit is one of thousands that plaintiffs have filed over the device, chiefly over side effects related to IUD uterine perforation and migration associated with Mirena.
Over 1,000 lawsuits have been consolidated as part of MDL No. 2434, the multidistrict litigation established in 2013 in the federal court of the Southern District of New York. On the state level, a mass tort has been established in Bergen County Superior Court, in New Jersey, which also includes over 1,000 cases.
In addition to the migration/perforation cases, a small number of lawsuits have also been filed over a neurological condition known as Idiopathic Intracranial Hypertension (or IIH) or pseudotumor cerebri (PTC), which the complaints allege are a side effect of the Mirena IUD.
- Newsweek, The Courtroom Controversy Behind Popular Contraceptive Mirena http://www.newsweek.com/2014/05/02/courtroom-controversy-behind-popular-contraceptive-mirena-248443.html
- NJ Courts, Mirena https://www.judiciary.state.nj.us/mass-tort/mirena/index.htm
- US District Court, Southern District of New York, In re: Mirena IUD Products Liability Litigation http://www.nysd.uscourts.gov/mdl/13MD02434
- Mayo Clinic, Pseudotumor-cerebri http://www.mayoclinic.org/diseases-conditions/pseudotumor-cerebri/basics/definition/con-20028792


 Resources
Resources
 Resources
Resources